Introduction
Genicular nerve radiofrequency ablation is a useful alternative to surgery in patients suffering from chronic pain due to moderate to severe osteoarthritis. Radiofrequency thermocoagulation of the genicular nerves has been shown to significantly reduce pain scores at 4 and 12 weeks of treatment (1).
Radiofrequency ablation (RFA) dates back to 1931 when it was used for the ablation of the Gasser ganglion for the treatment of trigeminal neuralgia (2). RFA generates a high-frequency electric current at the tip of the electrode toward a return plate connected to the body (3). This creates an electromagnetic field at the tip of an electrode inserted in the body, causing high-frequency ionic vibration. This phenomenon generates heat by friction at the cellular level, denaturing proteins and producing coagulation necrosis (4). The therapeutic efficacy of RFA depends on the size of the electrode, the temperature, the duration of the procedure, and the proximity and alignment of the electrode tip with the tissue of interest (5).
Knee osteoarthritis (OA) is a degenerative joint disease characterized by progressive loss of cartilage, alterations in the synovial membrane, and decreased viscosity of synovial fluid. This condition leads to pain, limited mobility, disability, and reduced quality of life (6). Its prevalence ranges from 15 % to 18 %, and it has been reported that 22 % of the U.S. population and 33 % of adults in the UK experience gonalgia (7).
Choi et al. in 2011 described a technique for the ablation of genicular nerves guided by fluoroscopy and evaluated its effect on knee pain in patients with OA. Of a total of 176 patients, 138 were excluded for not meeting inclusion criteria or declined to participate, and 38 were randomized and assigned to two groups: 19 to receive RFA and 19 as a control group. The technique performed under fluoroscopy involved placing a radiofrequency cannula at the junction of the diaphysis and femoral condyle in an AP (anteroposterior) view. The lesion was made with a 10 cm long, 22 G cannula, with a 10 mm active tip, in the posterior third of the femur and in a lateral view. A lesion was created at 70°C for 90 seconds. Follow-up evaluations were conducted at weeks 1, 4, and 12. They found that 59% of the RFA-treated group experienced a reduction in pain of at least 50%. Additionally, the RFA group showed functional improvement, with higher Oxford scale scores (35.9 points difference) compared to the control group. The authors thus demonstrated the utility of RFA for the management of knee OA. The basis of this technique is founded on anatomical studies of cadavers, where the constant site of appearance of genicular nerves was located at the previously described junction for the superomedial, inferomedial, and superolateral genicular nerves (8).
Anuj Bhatia et al., in 2016, published a review of studies on radiofrequency denervation of the knee. Out of 53 studies conducted between 2008 and 2015, only 13 were included in the analysis, and only two studies were randomized. These two studies demonstrated the effectiveness of RFA for pain reduction, improving functionality, without serious adverse effects (9).
In 2017, Jorge M. Orduña Valls et al. conducted a cadaveric study with 25 specimens, using ultrasound to determine possible anatomical variants of knee innervation. The conclusion of this study confirmed the sensory innervation of the knee and identified specific anatomical correlations that helped identify the nerves involved in knee innervation. These locations were subsequently corroborated by applying India ink to the structures using ultrasound guidance. The authors concluded that sensory innervation of the knee comes from various inconsistent branches of the sciatic, femoral, and obturator nerves. Medial innervation of the knee originates from the medial vastus nerve, the infrapatellar branch of the saphenous nerve, and the anterior branch of the obturator nerve. Lateral innervation comes from the intermediate vastus nerves, common peroneal nerve, and the lateral retinaculum (10).
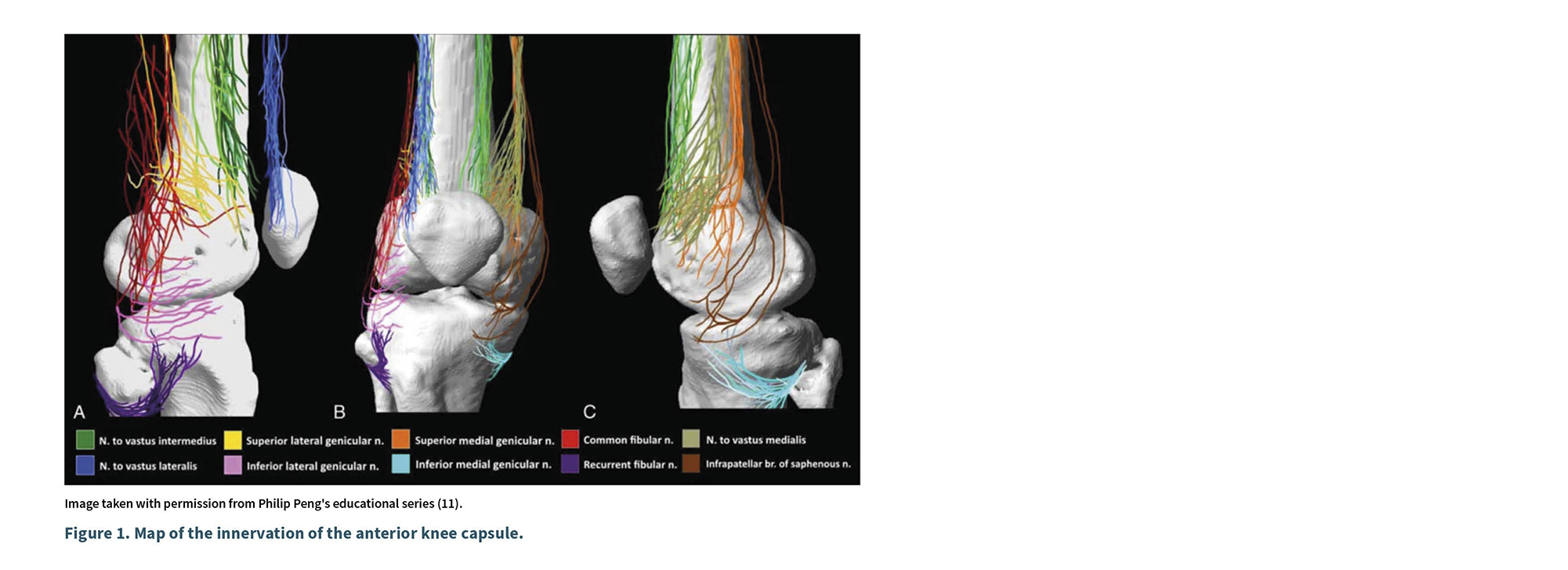
Philip Peng (2018) conducted a cadaveric study mapping the innervation of the knee and compared it with previous studies. He studied 15 cadavers with an average age of 80 years; dissected from its origin the medial vastus nerve (MVN), lateral nerve (LN), intermediate nerve (IN), saphenous nerve (SN), common fibular nerve (CFN), recurrent fibular nerve (RFN), obturator nerve (ON), and the genicular nerves, which included the superolateral genicular nerve (SLGN), inferolateral genicular nerve (ILGN), superomedial genicular nerve (SMGN), and inferomedial genicular nerve (IMGN) (11). He found that the knee is innervated by the VIN, NVLN, NVMN, SLGN, ILGN, SMGN, IMGN, CFN, RFN, and the infrapatellar branch of the saphenous nerve, which was primarily cutaneous. These articular branches ended in each of the four quadrants of the anterior capsule, with minimal overlap. The lower quadrants received fewer branches compared to the upper quadrants (11).
Peng found that the anterior capsule of the knee is invariably innervated by articular branches distributed in four quadrants as shown in Figure 1: superolateral quadrant (VLN, VIN, SLGN, and FCN); inferolateral quadrant (ILGN, RFN); superomedial quadrant (NVM, NVI, SMGN); and inferomedial quadrant (IMGN and IPBSN). All articular branches, except NVM and NVL, traverse the periosteum up to 0.9 mm from it (11).
Clinical case
A 65-year-old man with a history of diabetes, hypertension, hypertensive heart disease, and overweight (BMI 29), with no allergies or previous surgeries, presented with a four-year history of right knee pain. The pain, characterized as sharp, had an intensity of 6/10 at rest and 8/10 during activity, localized in the anteromedial region and not irradiating to the upper or lower lateral regions, limiting his daily activities such as driving, walking for 15 minutes, or using public transport. The patient also reported morning stiffness and poor response to non-steroidal anti-inflammatory drugs (NSAIDs). The pain improved with rest and had a minimal response to NSAIDs; it interfered with sleep and was associated with a decrease in knee strength. The evaluation using the Oxford scale was 12 points, interpreted as severe OA.
On physical examination, swelling due to joint hypertrophy was evident, and palpation of the joint line elicited pain. The patient hadknee stiffness, decreased range of motion during passive mobilization, and crepitus during flexion. No pain was detected upon palpation of the anserine bursa or the iliotibial band. Ultrasound revealed the formation of osteophytes in the medial region, synovial hypertrophy, decreased joint space, reduced thickness of hyaline cartilage, and a mild joint effusion. Anteroposterior (AP) and lateral radiographs showed grade IV OA according to Kellgren-Lawrence classification. Initially, paracetamol and NSAIDs were prescribed without response, leading to a referral to the Pain Management Center. Due to the history of comorbidities, an interventional pain management approach was chosen based on genicular nerve blocks (superomedial, inferomedial, superolateral) with a 70 % reduction in pain. Subsequently, radiofrequency ablation of the articular nerve branches of the knee was planned.
Technique description
Prior to obtaining informed consent for the procedure and publication of the case with photographs, the patient was placed in a supine position in the intervention room, with standard monitoring according to the guidelines of the American Society of Anesthesiologists (ASA). The right knee was positioned with a 20° flexion and externally rotated. The return plate of the radiofrequency generator was placed on the posterior thigh, and sterile preparation of the knee was performed, from the thigh to the leg, using povidone. Sterile drapes were placed, corresponding medications were prepared, and the ultrasound transducer was covered with a sterile sheath.
A diagnostic scan was performed with a linear transducer (10-12 MHz Sonosite Px) in nerve imaging mode. The examination began at a depth of 4 cm, with a resolution of 12 MHz, starting from the axis of the femur in the medial region and extending to the junction with the femoral condyle (epicondyle). Color Doppler was activated to identify the superomedial genicular artery; subsequently, the transducer was rotated to obtain a short-axis view. In that position (junction of the diaphysis and metaphysis), the distal portion of the transducer was rotated caudally as shown in Figure 2. The skin and the trajectory of the cannula were infiltrated with lidocaine (1 % Pisacaína). An EVA© 18G, 10 cm, 10 mm active tip curved radiofrequency cannula was inserted from anterior to posterior, 2 cm from the transducer, ensuring the active tip remained parallel to the periosteum. A second skin infiltration was performed 1 cm from the transducer along the needle trajectory, and a second radiofrequency cannula was inserted parallel to the periosteum, from anterior to posterior.
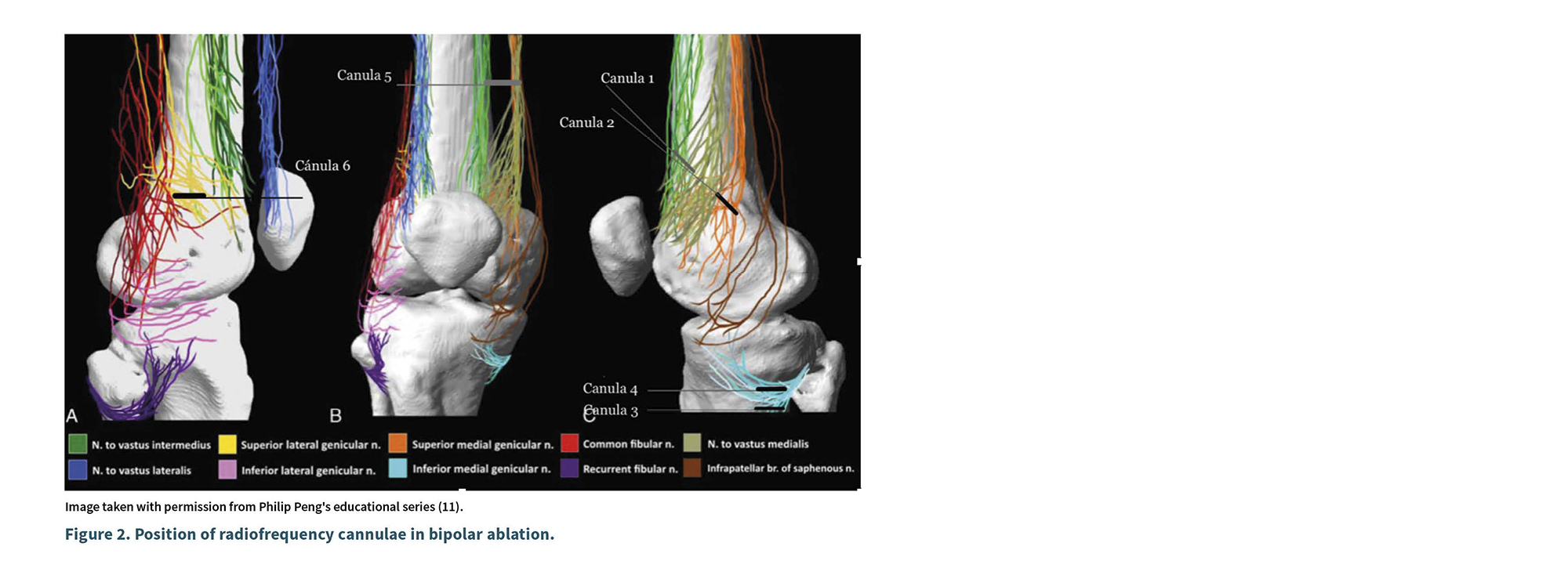
The final position of the second needle tip was located 2 cm distal to the tip of the first, maintaining both parallel to the periosteum at the edge of the posterior third of the femoral epicondyle in lateral view; this positioning places the tip of the first needle 1 cm from the start of the active tip of the second. A sensory stimulation was performed, starting with 0.3 V, 50 Hz, and 2 ms, until the patient perceived paresthesia; then, the voltage for sensory stimulation was doubled to 2 V, 2 Hz, and 2 ms, with no motor response observed. The ablation areas were anesthetized with 1 % lidocaine (1 ml per cannula), and radiofrequency ablation was performed using the Boston Scientific G4 equipment in bipolar mode at 70 ºC for 90 seconds in 2 cycles.
The second lesion (cannulas 3 and 4) was performed in the inferomedial region, in the sagittal axis of the tibia. For this, a scan was conducted following the trajectory of the medial collateral ligament, at the junction of the diaphysis and metaphysis of the tibia, and the inferomedial genicular artery was identified using color Doppler. Two ml of local anesthesia were infiltrated into the skin and along the needle pathway, and access was obtained outside the imaging plane from anterior to posterior using an EVA© 10 cm, 18 G, 10 mm active tip curved radiofrequency cannula until the tip of the needle was positioned at the junction of the mentioned structures, 7 mm in a cephalic direction relative to the first needle; the skin was infiltrated, and a second radiofrequency cannula was punctured, located 7 mm cephalically from the first. Both cannulas were verified in the transverse axis and repositioned so that their active tips remained parallel to the periosteum in the posterior third. A sensory stimulation was performed starting with 0.3 V, 50 Hz, and 2 ms until the patient perceived paresthesia; then, the voltage for motorstimulation was doubled to 2 V, 2 Hz, and 2 ms, with no motor response observed. The area was infiltrated with 1 % lidocaine (1 ml per needle), and after allowing for latency, a bipolar lesion was created at 80 °C for 90 seconds (25-26 V in two cycles).
Third Access Point (Cannula 5): At 15 cm from the joint line, the transducer was positioned in the transverse plane of the femur, in the medial region, identifying the sartorius muscle, the medial vastus, and the long adductor; between the fascia of the sartorius and that of the medial vastus, the hyperechoic image of the medial vastus nerve was identified. After infiltrating the skin with 1 % lidocaine, access was obtained from anterior to posterior in the same plane using a 20 G radiofrequency cannula with a 10 mm active tip, curved. Sensory stimulation was applied starting at 0.3 V until an anteromedial response from the knee was obtained; the stimulation was repeated at 2 Hz and 0.7 V, until contraction of the medial vastus muscle was evidenced. A pulsed radiofrequency cycle was applied for 420 seconds at 42 °C, 55 V, and 10 ms, at 5 Hz. Upon completion, 2 ml of 1 % lidocaine were administered, and the technique was concluded.
Post-procedure follow-up was conducted at 2, 4, and 12 weeks, allowing for the observation of a reduction in pain to 2/10 on the visual analog scale of pain; this improvement was maintained at 12 weeks. The Oxford scale at 4 and 12 weeks was 38 points, and the WOMAC score was 33 points at 12 weeks (prior to the intervention, 93 points). Therefore, there was an improvement in stiffness and functional difficulty. Additionally, pharmacological therapy continued, limited to paracetamol as needed.
Discussion
Knee degenerative disease is a common condition in individuals over 60 years of age; pain management is often frustrating as currently described techniques are not completely effective and, in some cases, are inaccessible. Although surgery is considered the definitive treatment for this pathology, not all patients are candidates due to pre-existing comorbidities, and many continue to experience pain even after total replacement.
Before the anatomical studies conducted by Dr. Peng in 2018, little was known about the innervation of the knee joint, and previously described techniques considered a single therapeutic point for each anatomical region. However, detailed cadaveric studies have shown that knee innervation is considerably more complex and generally involves more than three nerve branches. Peng (11)demonstrated that in each quadrant, the knee is innervated by at least three nerve branches, essentially forming a network that traverses the periosteum in each quadrant. Only two branches emerge from the periosteum and can fortunately be addressed before they branch into their terminal branches.
Current techniques using monopolar radiofrequency ablation tend to be insufficient for capturing nerve branch innervation due to the complex innervation described previously (12-15).
Recently, cooled radiofrequency ablation has been described, a technology that allows for the generation of a larger spherical lesion, thereby covering a greater ablation area and consequently capturing a larger number of nerve endings, making it an attractive alternative for pain management in OA. While this technique has demonstrated effectiveness in some long-term studies, it tends to be costly and difficult to access for the general population (16).
Ashok (2018) conducted a comparative study between monopolar and bipolar radiofrequency, utilizing a fluoroscopy-guided technique. This study evidenced that the bipolar technique is an effective alternative for ablating genicular nerves in patients with knee pain secondary to OA. The bipolar technique showed better pain control from the first month up to the sixth month of follow-up, with this difference being statistically significant. Additionally, patients in the radiofrequency group had higher scores on the Oxford scale at 6 months; however, this difference was not statistically significant (17).
The technique described in this report incorporates some modifications adapted to the recently described anatomical scheme. Firstly, it is performed using ultrasound, allowing for manipulation of the cannula to ensure it is positioned parallel to the periosteum at the previously defined target. Furthermore, bipolar lesions have a different morphology depending on the placement of the needle. Typically, if the cannulas are placed parallel to each other and the distance between the tips does not exceed 1 cm, a square lesion of 1 cm will be created; while this finding is attractive, given the anatomical arrangement described by Peng (8), the lesion should be larger to encompass the circumference of the epicondyle. Our technique allows for the creation of a rhomboidal or linear lesion of 2.5 to 3 cm on the periosteum, with a width of 5 to6 mm, effectively coagulating the anatomical innervation network (Figure 2). Additionally, considering that the bipolar technique described by Ashok (2018) is superior to the monopolar technique, it should be noted that the cannulas in Ashok’s study were aligned along the axis of the femur, whereas in the technique presented in this report, the cannulas are positioned in the transverse axis: one in an anterior position and the other in a more posterior position, which is anatomically consistent as shown in Figures 3 and 4.
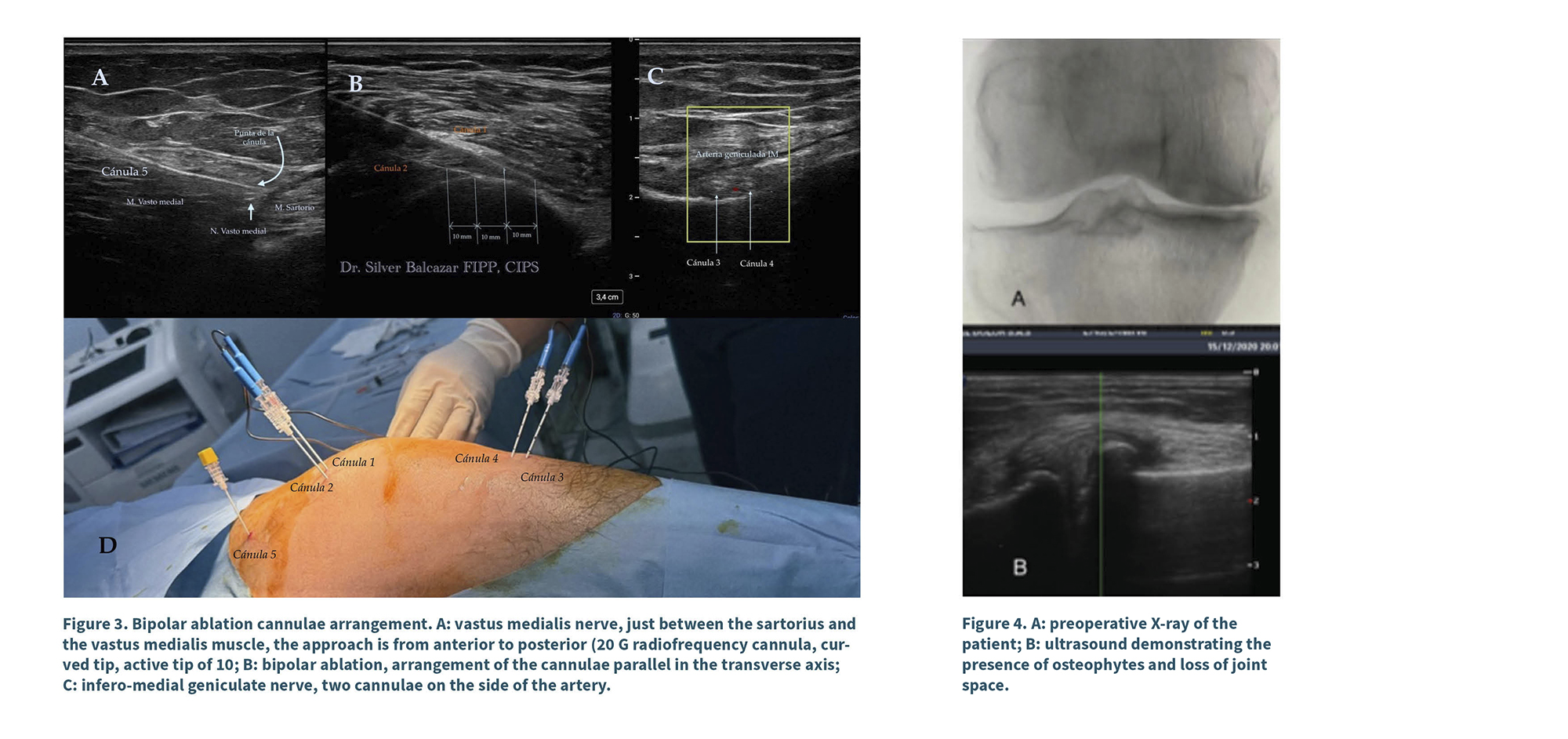
Before conducting the technique on patients, experimental tests were performed on chicken cadaveric tissue (chicken breast) to observe the lesion configuration in each technique—monopolar, bipolar, and modified bipolar—where differences were found both in the position of the cannulas and the size of the lesion (Figure 5). This allowed us to determine the correct placement of the cannulas to achieve an adequate lesion size.

The results in the patient presented here are very promising at the 12-week follow-up, and no adverse effects occurred other than those described in previous techniques. This technique was made possible thanks to the current understanding of knee innervation and the optimization of cannula positioning. However, since this is a case report, larger case series and comparative studies are required to determine whether this technique is superior to traditional fluoroscopic techniques or to the monopolar techniques described using ultrasound.
Conclusion
The modified bipolar radiofrequency ablation technique guided by ultrasound represents a promising advance in the interventional pain management of patients with severe knee osteoarthritis. Unlike traditional monopolar techniques, this approach considers the complex innervation of the knee as described in recent anatomical studies, particularly the findings of Peng.
The modification of the traditional bipolar technique, positioning the cannulas in the transverse axis and parallel to the periosteum, allows for the creation of a larger lesion that more efficiently encompasses the innervation network in each quadrant. The use of ultrasound as guidance enhances precision in cannula placement and increases the safety of the procedure.
The results obtained in this case show significant and sustained improvement in pain and functionality at 12 weeks, suggesting that this technique could be an effective alternative for patients who do not respond to conservative treatments and are not surgical candidates. However, comparative studies with a larger number of patients are needed to establish the superiority of this technique over established methods.
Conflict of interest
None.
Funding
None.
references
1. Iannaccone F, Dixon S, Kaufman A. A Review of Long-Term Pain Relief after Genicular Nerve Radiofrequency Ablation in Chronic Knee Osteoarthritis. Pain Physician. 2017;20(3):E437-E444.
2. Aggarwal AK, Ottestad E, Pfaff KE, Huai-Yu Li A, Xu L, Derby R, ET AL. Review of Ultrasound-Guided Procedures in the Management of Chronic Pain. Anesthesiol Clin. 2023;41(2):395-470.
3. Emril DR, Ho KY. Treatment of trigeminal neuralgia: role of radiofrequency ablation. J Pain Res. 2010;3:249-54.
4. Liu YC, Zhao XL, Zhou JX, Dou CY, Zhang YJ. Radiofrequency ablation therapy for knee osteoarthritis: a systematic review and meta-analysis. Cir Cir. 2024;92(4):456-68.
5. Ward E, Munk PL, Rashid F, Torreggiani WC. Musculoskeletal interventional radiology: radiofrequency ablation. Radiol Clin North Am. 2008;46(3):599-610. DOI: 10.1016/J.RCL.2008.02.006
6. Imani F, Patel VB. Therapeutic Challenges for Knee Osteoarthritis. Anesth Pain Med. 2019;9(3).
7. Damen J, van Rijn RM, Emans PJ, Hilberdink WKHA, Wesseling J, Oei EHG, et al. Prevalence and development of hip and knee osteoarthritis according to American College of Rheumatology criteria in the CHECK cohort. Arthritis Res Ther. 2019;21(1):4.
8. Choi WJ, Hwang SJ, Song JG, Leem JG, Kang YU, Park PH, et al. Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain. 2011;152(3):481-7.
9. Bhatia A, Peng P, Cohen SP. Radiofrequency Procedures to Relieve Chronic Knee Pain: An Evidence-Based Narrative Review. Reg Anesth Pain Med. 2016;41(4):501-10.
10. Orduña Valls JM, Vallejo R, López Pais P, Soto E, Torres Rodríguez D, Cedeño DL, et al. Anatomic and Ultrasonographic Evaluation of the Knee Sensory Innervation: A Cadaveric Study to Determine Anatomic Targets in the Treatment of Chronic Knee Pain. Reg Anesth Pain Med. 2017;42(1):90-8.
11. Tran J, Peng PWH, Lam K, Baig E, Agur AMR, Gofeld M. Anatomical Study of the Innervation of Anterior Knee Joint Capsule: Implication for Image-Guided Intervention. Reg Anesth Pain Med. 2018;43(4):407-14.
12. Kim DH, Lee MS, Lee S, Yoon SH, Shin JW, Choi SS. A Prospective Randomized Comparison of the Efficacy of Ultrasound- vs Fluoroscopy-Guided Genicular Nerve Block for Chronic Knee Osteoarthritis. Pain Physician. 2019;22(2):139-46.
13. Kim DH, Choi SS, Yoon SH, Lee SH, Seo DK, Lee IG, et al. Ultrasound-Guided Genicular Nerve Block for Knee Osteoarthritis: A Double-Blind, Randomized Controlled Trial of Local Anesthetic Alone or in Combination with Corticosteroid. Pain Physician. 2018;21(1):41-52.
14. El-Hakeim EH, Elawamy A, Kamel EZ, Goma SH, Gamal RM, Ghandour AM, et al. Fluoroscopic Guided Radiofrequency of Genicular Nerves for Pain Alleviation in Chronic Knee Osteoarthritis: A Single-Blind Randomized Controlled Trial. Pain Physician. 2018;21(2):169-77.
15. Jamison DE, Cohen SP. Radiofrequency techniques to treat chronic knee pain: a comprehensive review of anatomy, effectiveness, treatment parameters, and patient selection. J Pain Res. 2018;11:1879-88.
16. Desai M, Bentley A, Keck WA, Haag T, Taylor RS, Dakin H. Cooled radiofrequency ablation of the genicular nerves for chronic pain due to osteoarthritis of the knee: a cost-effectiveness analysis based on trial data. BMC Musculoskelet Disord. 2019;20(1).
17. Jadon A, Jain P, Motaka M, Swarupa CP, Amir M. Comparative evaluation of monopolar and bipolar radiofrequency ablation of genicular nerves in chronic knee pain due to osteoarthritis. Indian J Anaesth. 2018;62(11):876-80.